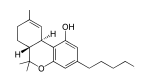
Tetrahydrocannabinol
 | |
 | |
| Datos Klinikal | |
|---|---|
| Kategorya sa pagdadalangtao |
|
| Dependence liability | 8–10%[1] |
| Mga ruta ng administrasyon | orally, smoked (or vaporized) |
| Kodigong ATC | |
| Estadong Legal | |
| Estadong legal |
|
| Datos Parmakokinetiko | |
| Bioavailability | 10–35% (inhalation), 6–20% (oral)[2] |
| Pagbuklod ng protina | 95–99%[2] |
| Metabolismo | mostly hepatic by CYP2C[2] |
| Biyolohikal na hating-buhay | 1.6–59 h,[2] 25–36 h (orally administered dronabinol) |
| Ekskresyon | 65–80% (feces), 20–35% (urine) as acid metabolites[2] |
| Mga pangkilala | |
| |
| Singkahulugan | Dronabinol |
| Bilang ng CAS | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.153.676 |
| Datos Kemikal at Pisikal | |
| Pormula | C21H30O2 |
| Bigat Molar | 314.4636 |
| Modelong 3D (Jmol) | |
| Tiyak na pihit | -152° (etanol) |
| Punto ng pagkulo | 157 °C (315 °F) [4] |
| Pagkatunaw sa tubig | 0.0028[3] (23 °C) mg/mL (20 °C) |
| |
| |
| | |
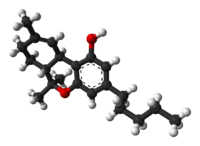
Ang Tetrahydrocannabinol ( /ˌtɛtrəˌhaɪdrɵkəˈnæbɪnɔːl/ tet-rə-HY-drə-kə-NAB-i-nawl or /ˌtɛtrəˌhaɪdrɵkəˈnæbɪnɒl/ tet-rə-HY-drə-kə-NAB-i-nol;[5] THC), o mas tumpak ay ang pangunahing isomer (−)-trans-Δ9-tetrahydrocannabinol ((6aR,10aR)-delta-9-tetrahydrocannabinol) ang pangunahing sangkap na sikoaktibo o cannabinoid ng halamang cannabis o marijuana. Ito ay unang ihiniwalay noong 1964 ng mga siyentipikong Israeli na sina Raphael Mechoulam, Yechiel Gaoni at mga kasama nito sa Hebrew University of Jerusalem.[6][7][8] Ang isang pormulasyong pharmaceutical ng (−)-trans-Δ9-tetrahydrocannabinol na kilala sa INN dronabinol ay makukuha sa pamamagitan ng pagrereseta sa Estados Unidos at Canada sa ilalim ng pangalang Marinol. Ito ay isang aromatic terpenoid na may napakababang solubilidad sa tubig ngunit mabuting solubilidad sa karamihan ng mga organikong solvent sa spesipiko ay mga lipid at alkohol.[3]
Ang mga aksiyong parmakolohikal nito ay nagreresulta mula sa parsiyal na gawaing agonista sa cannabinoid receptor CB1 (Ki=10nM[13]) na matatagpuan sa sentral na sistemang nerbiyos at CB2 receptor (Ki=24nM[14]) na pangunahing inahahayag sa mga selula ng sistemang immuno. Ang mga epektong sikoaktibo nito ay pangunahing pinamamagitan ng aktibasyon ng mga CB1G-protein coupled receptor na nagpapabawas ng konsentrasyon ng ikalawang mensaherong molekulang cAMP sa pamamagitan ng pagpigil ng adenylate cyclase.
Ang THC ay may mga epektong analhesiko at ang marijuana ay magagamit upang gamutin ang kirot sa pamamagitan ng pagbabago ng paglabas ng transmitter sa dorsal root ganglion ng spinal cord at periaqueductal gray.[9] Ang ibang mga epekto nito ay kinabibilangan ng relaksasyon, pagbabago ng mga pandamang paningin, pandinig at pang-amoy, at stimulasyon ng gana sa pagkain. Ang THC ay mga katangiang antiemetiko at maaring magbawas ng agresyon sa ilang mga indibidwal.[10]
Isomerism[baguhin | baguhin ang wikitext]

| 7 double bond isomers at kanilang 30 stereoisomer | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pagbibilang na Dibenzopyran | Pagbibilang na Monoterpenoid | Bilang ng mga stereoisomer | Pag-iral sa kalikasan | Convention on Psychotropic Substances Schedule | Istuktura | |||
| Maikling pangalan | Mga Chiral center | Buong pangalan | Maikling pangalan | Mga Chiral center | ||||
| Δ6a,7-tetrahydrocannabinol | 9 and 10a | 8,9,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ4-tetrahydrocannabinol | 1 at 3 | 4 | Hindi | Schedule I | 
|
| Δ7-tetrahydrocannabinol | 6a, 9 and 10a | 6a,9,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ5-tetrahydrocannabinol | 1, 3 at 4 | 8 | Hindi | Schedule I | 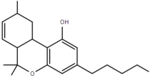
|
| Δ8-tetrahydrocannabinol | 6a and 10a | 6a,7,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ6-tetrahydrocannabinol | 3 at 4 | 4 | Oo | Schedule I | 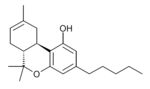
|
| Δ9,11-tetrahydrocannabinol | 6a and 10a | 6a,7,8,9,10,10a-hexahydro-6,6-dimethyl-9-methylene-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ1,7-tetrahydrocannabinol | 3 and 4 | 4 | Hindi | Schedule I | 
|
| Δ9-tetrahydrocannabinol | 6a and 10a | 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ1-tetrahydrocannabinol | 3 and 4 | 4 | Oo | Schedule II | 
|
| Δ10-tetrahydrocannabinol | 6a and 9 | 6a,7,8,9-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ2-tetrahydrocannabinol | 1 and 4 | 4 | Hindi | Schedule I | 
|
| Δ6a,10a-tetrahydrocannabinol | 9 | 7,8,9,10-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ3-tetrahydrocannabinol | 1 | 2 | Hindi | Schedule I | 
|
Mga sanggunian[baguhin | baguhin ang wikitext]
- ↑ "Based upon several nationwide epidemiological studies, marijuana’s dependence liability has been reliably determined to be 8 to 10 percent." Douglas B. Marlowe, J.D., Ph.D. (December 2010). "The Facts On Marijuana". NADCP.
{{cite journal}}: Cite journal requires|journal=(tulong)CS1 maint: multiple names: mga may-akda (link) - ↑ 2.0 2.1 2.2 2.3 2.4 Grotenhermen F (2003). "Pharmacokinetics and pharmacodynamics of cannabinoids". Clin Pharmacokinet. 42 (4): 327–60. doi:10.2165/00003088-200342040-00003. PMID 12648025.
- ↑ 3.0 3.1 Garrett, Edward R.; C. Anthony Hunt (1974). "Physicochemical properties, solubility, and protein binding of Δ9 -tetrahydrocannabinol". J. Pharm. Sci. 63 (7): 1056–64. doi:10.1002/jps.2600630705. PMID 4853640.
{{cite journal}}: Unknown parameter|month=ignored (tulong) - ↑ "Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?" (PDF). Inarkibo mula sa ang orihinal (PDF) noong 2017-06-22. Nakuha noong 2013-04-26.
- ↑ Dictionary Reference: tetrahydrocannabinol: /ˌtɛtrəˌhaɪdrəkəˈnæbəˌnɔːl/, /ʔˌnɒl/
- ↑ Gaoni, Y.; Mechoulam, R. (1964). "Isolation, structure and partial synthesis of an active constituent of hashish". Journal of the American Chemical Society. 86 (8): 1646–1647. doi:10.1021/ja01062a046.
- ↑ Interview with the winner of the first ECNP Lifetime Achievement Award: Raphael Mechoulam, Israel Naka-arkibo 2011-04-30 sa Wayback Machine. February 2007
- ↑ Geller, Tom (2007). "Cannabinoids: A Secret History". Chemical Heritage Newsmagazine. 25 (2). Inarkibo mula sa orihinal noong June 19, 2008. Nakuha noong September 29, 2013.
- ↑ PMID 11316486 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Hoaken (2003). "Drugs of abuse and the elicitation of human aggressive behavior". Addictive Behaviors. 28: 1533–1554.